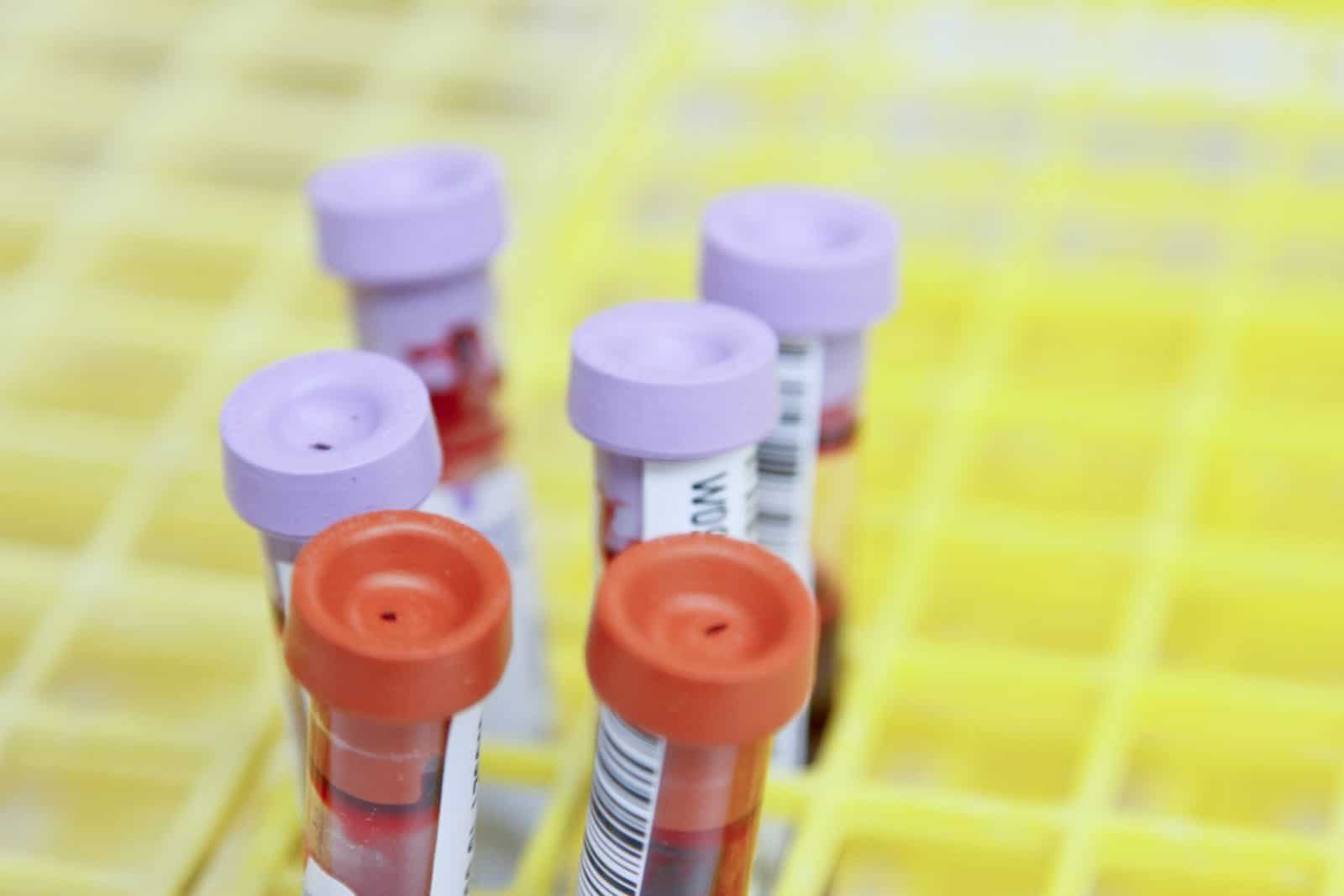
A recent study conducted at the Fred Hutchinson Cancer Center in Seattle, Washington, has unveiled promising results regarding the early detection of colorectal cancer (CRC). According to the study, a simple blood test has demonstrated an accuracy rate of over 80% in identifying CRC cases. The findings, published in The New England Journal of Medicine, involved nearly 8,000 participants aged between 45 and 84, shedding light on a potential breakthrough in cancer screening.
Comparing the results of the SHIELD blood test, developed by Guardant, a pharmaceutical company based in Palo Alto, California, with those of the conventional colonoscopy procedure—the gold standard for CRC screening—researchers found encouraging outcomes. Among participants diagnosed with colorectal cancer through colonoscopy, the blood test identified over 83% of cases, with a 16.9% rate of false negatives.
The effectiveness of the SHIELD test lies in its ability to detect signs of colorectal cancer through circulating tumor DNA (ctDNA) shed by tumors. While the test excelled in identifying colorectal cancers, it exhibited less efficiency in detecting precancerous lesions, as per the study’s findings.
Dr. William M. Grady, a gastroenterologist at the Fred Hutchinson Cancer Center and co-author of the study, emphasized that the SHIELD blood test is intended for average-risk individuals without symptoms. He compared its accuracy to at-home stool tests commonly used for early detection.
Despite the promising results, the study noted certain limitations. The test is not currently recommended for high-risk individuals, such as those with a family history of CRC or inflammatory bowel disease.
Colorectal cancer stands as the second leading cause of cancer-related deaths in the United States, projected to claim over 53,000 lives in 2024. The American Cancer Society recommends regular screenings starting at age 45 for individuals at average risk, underscoring the importance of early detection.
However, screening adherence remains a challenge, with 40% to 50% of eligible individuals not undergoing recommended screenings. Dr. Grady expressed hope that the introduction of a blood-based screening test could enhance screening rates, given its higher acceptability among the population.
Nevertheless, some medical experts have raised concerns regarding the test’s effectiveness, particularly its lower sensitivity to precancerous symptoms compared to existing screening methods like Cologuard. The American Gastroenterological Association acknowledged the potential of blood-based tests in identifying colorectal cancer early but cautioned against relying solely on them, emphasizing the necessity of colonoscopies for detecting precancerous polyps.
Despite these considerations, the SHIELD blood test presents a promising addition to existing screening methods for colorectal cancer. While it may not replace traditional screening approaches, its introduction could offer an additional tool for early detection, potentially saving lives in the process. Individuals interested in the SHIELD blood test are advised to consult with their primary care providers, particularly those hesitant to undergo conventional screening methods. With colorectal cancer being largely preventable, advancements in screening technologies like the SHIELD blood test offer hope in combating this deadly disease.
New Blood Test Shows Promise in Detecting Colorectal Cancer by Bangkok Health Service
Read more about cancer:
The Importance of Early Health Checks for Families with a History of Colorectal Cancer
Understanding Cancer: Insights from the 2025 Cancer Facts and Figures Report
Understanding Cancer: Insights from the 2024 Cancer Facts and Figures Report
Get a cancer screening with Bangkok Health Service:
Cancer Screening in Bangkok – Basic, Advanced & Early Detection 2 nights Packages
Cancer Screening Packages in Bangkok | Early Detection & Affordable Tests